'Netanyahu planning attack?'
Russian paper quotes 'informed' Israeli source as saying PM's secret visit to Moscow may have been in order to inform Kremlin that 'Israel may be ready to move on to decisive actions with regards to Iran' Full Story . . .
And he causeth all, both small and great, rich and poor, free and bond, to receive a mark in their right hand, or in their foreheads: And that no man might buy or sell, save he that had the mark, or the name of the beast, or the number of his name. (Revelation 13:16)
This Health Care Package (HCP) being pushed by the president includes the following:
July 14, 2009 (12:51 p.m.)
F:\P11\NHI\TRICOMM\AAHCA09_001.XMLf:\VHLC\071409\071409.140.xml
http://waysandmeans.house.gov/media/...CA09001xml.pdf
page 503
1(B) conduct and support systematic re2views of clinical research, including original re3search conducted subsequent to the date of the4 enactment of this section;
5 (C) continuously develop rigorous sci6entific methodologies for conducting compara7tive effectiveness studies, and use such meth8odologies appropriately;
9 (D) submit to the Comparative Effective10ness Research Commission, the Secretary, and11 Congress appropriate relevant reports described12 in subsection (d)(2); and
13 (E) encourage, as appropriate, the devel14opment and use of clinical registries and the de15velopment of clinical effectiveness research data16 networks from electronic health records, post17 marketing drug and medical device surveillance18 efforts, and other forms of electronic health19 data.
--------------------------------------------------------------------------
page 1006
trends, adverse event patterns, incidence and preva2lence of adverse events, and other information the3 Secretary determines appropriate, which may include4 data on comparative safety and outcomes trends;5 and6 ‘‘(E) shall establish procedures to permit public7 access to the information in the registry in a manner8 and form that protects patient privacy and propri9etary information and is comprehensive, useful, and10 not misleading to patients, physicians, and sci11entists.12 ‘‘(5) To carry out this subsection, there are author13ized to be appropriated such sums as may be necessary14 for fiscal years 2010 and 2011.’’.15
(2) EFFECTIVE DATE.—The Secretary of16 Health and Human Services shall establish and17 begin implementation of the registry under section18 519(g) of the Federal Food, Drug, and Cosmetic19 Act, as added by paragraph (1), by not later than20 the date that is 36 months after the date of the enactment of this Act, without regard to whether or22 not final regulations to establish and operate the23 registry have been promulgated by such date.A medical device surveillance within three years, no matter what!
Sounds like...........
Thursday, September 10, 2009
Subscribe to:
Post Comments (Atom)
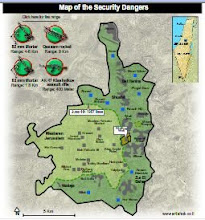
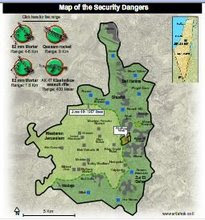
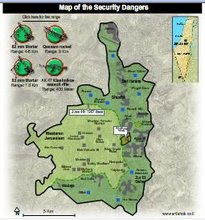
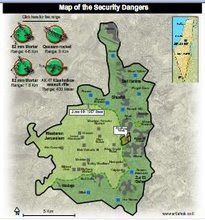
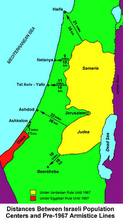
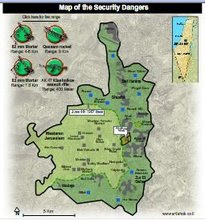
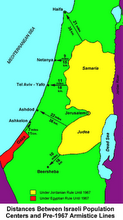
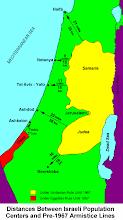
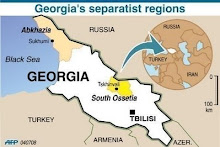
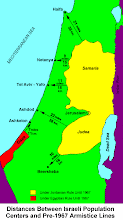
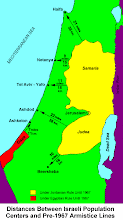
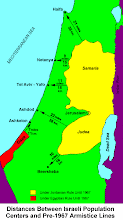
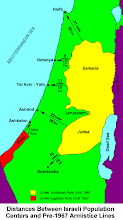
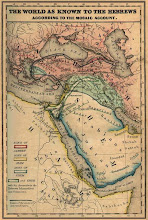
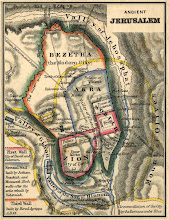
Divided Jerusalem






































































































+and+Iran%27s+Ahmadinejad.jpg)






































































.jpg)




































.jpg)





.jpg)
















































































































































.jpg)




























































































+and+FM+Livni.jpg)





















































































































No comments:
Post a Comment